Platonic Solids, Chemical Explosives, and the World’s Smallest Helium Balloon
Fields covered: Chemistry, Organic Synthesis, Geometry, Energetic Materials
Related Articles:
§ ![]() Cubane: 50 Years Later §
Cubane: 50 Years Later § ![]() Cubane §
Cubane § ![]() Octanitrocubane: A New Nitrocarbon §
Octanitrocubane: A New Nitrocarbon § ![]() Towards dodecahedrane §
Towards dodecahedrane §![]() Dodecahedrane §
Dodecahedrane §![]() Putting Helium Inside Dodecahedrane
Putting Helium Inside Dodecahedrane
Regular polygons, such as squares and equilateral triangles, are flat shapes with equal side lengths and angles. Put six squares together and you get a cube, a solid with identical regular polygons for faces and the same number of faces around each corner. It turns out that only five such solids exist: the cube, tetrahedron, octahedron, dodecahedron, and icosahedron. These are known as the Platonic solids, named after the ancient Greek philosopher Plato, who theorised that these five solids constituted everything in the universe.
Unfortunately for Plato, scientists eventually discovered that atoms, not Platonic solids, made up everything in the universe (well, matter at least). But not long after, some other scientists began to wonder whether atoms could also make up the Platonic solids themselves. They set to work, fixing carbon atoms at corners and adding hydrogen atoms where needed. In the end, two molecular analogues of the Platonic solids were made, and they came to be known as the Platonic hydrocarbons.
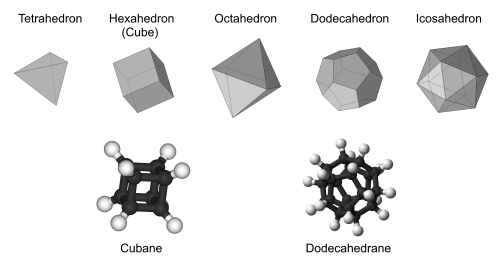
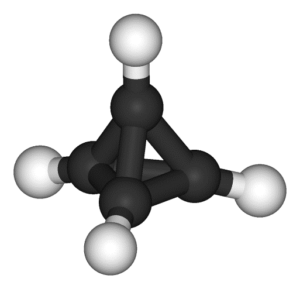
In 1964, cubane became the first Platonic hydrocarbon to be successfully synthesised. As its name suggested, it had a cubic arrangement of eight carbon atoms, all bonded at right angles (90°) to each other. The molecule itself was surprisingly stable, as carbon atoms naturally prefer to have bonds 109.5° apart. Like a tightly compressed spring, this caused the strained bonds of cubane to store a lot of energy, just waiting to be released.
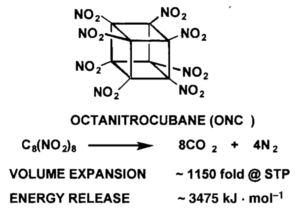
Chemists exploited this property by replacing all of cubane’s hydrogen atoms with nitro groups, the chemical motif that makes TNT such a blast. The result was octanitrocubane, a chemical explosive that detonated at over 10000 m/s, generating 12 equivalents of hot carbon dioxide and nitrogen gas. Overall, octanitrocubane packed more than twice its weight in TNT, making it one of the most powerful non-nuclear explosives ever produced.
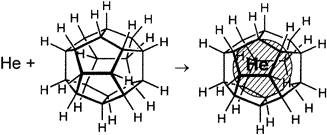
The other Platonic hydrocarbon was dodecahedrane, first synthesised in 1982. The bonds of dodecahedrane were 108° apart, which was very close to the ideal angle of 109.5°. This meant that unlike cubane, dodecahedrane lacked any strained bonds or explosive properties. What it did have was a relatively spacious interior, potentially just large enough to fit something inside.
And so, like the proverbial cat that sits, some scientists proceeded to shoot a focused beam of high-energy helium ions at a sample of dodecahedrane. A tiny fraction of those helium ions managed to slip through the bonds and get trapped inside the dodecahedrane molecule. This technique effectively produced the world’s smallest helium balloons, each containing just one helium atom.
Today, three Platonic solids remain without molecular analogues. Unfortunately, both octahedrane and icosahedrane lie just beyond the realm of possibility, with the former requiring all four carbon bonds on the same side and the latter needing more bonds (five) than carbon can make.
On the other hand, tetrahedrane continues to be merely improbable, and chemists continue to attempt its synthesis. Only time will tell if their efforts are successful and what surprises the last Platonic hydrocarbon has in store for us.