Magic Bullet? No more.
Fields covered: Anti-Microbial Resistance, Phage Therapy, Microbiology
Updated Daily!
A Brief History of Phage Therapy and Antibiotics
More than a century ago, a French microbiologist named Félix d’Hérelle noticed that some type of “disease” was spreading through his bacteria culture. Little was known about viruses back then and Félix concluded that a virus named “bacteriophage” – or “phage” for short – was preying on the bacteria. He then isolated these phages to treat children with dysentery, an intestinal infection (Landecker,2016). Despite promising results, further developments in phage therapy were neglected after the accidental discovery of penicillin, an antibiotic by Alexander Fleming in 1928.
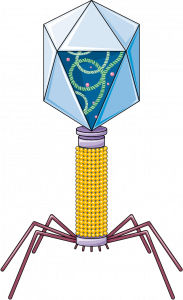
After the discovery of penicillin, it seemed as though humanity had finally overcome the threat of bacterial infections and that these diseases were a thing of the past. Antibiotics were then touted as the “magic bullet” against any bacterial infections. However, as early as 1947, two years after penicillin had been commercialized, bacteria resistant to the effect of penicillin was discovered. This was a result of selective pressure acting on bacteria as bacteria resistant to the antibiotic treatment were able to pass their genes on to the next generation. Evidently, the dwindling effectiveness of current antibiotic treatments poses an ever-growing challenge for hospitals worldwide.
The Advantage of Using Therapeutic Phages
The biggest edge therapeutic phages have over conventional antibiotics is the bacterial specificity. Phages have tail fibres that help them anchor onto the bacteria surface before injecting their DNA into them. These tail fibres are specific to antigens present on the bacterial cell. This means that we could potentially customize a cocktail of phages to combat a specific infection without harming the normal flora of the human body. Although phages have the upper hand in terms of bacterial specificity, it is a double-edged sword. The specificity of a bacteriophage makes the identification of therapeutic phages a long and tedious process, but some patients do not have the benefit of time. Phages are also bacteria strain-specific. That means that one phage alone is unable to infect a whole species of bacteria; rather, it is only effective against certain strains of bacteria.
Two is better than one
One might ask: if bacteria can develop antibiotics resistance, wouldn’t it be possible for them to develop phage resistance too? Well, bacteria can indeed develop phage resistance. However, there are two reasons why it shouldn’t be a big concern. Firstly, phages are ubiquitous, and have a higher replication rate compared to bacteria. This means that phages can acquire beneficial mutations more quickly. Secondly, antibiotics can work synergistically together to combat bacterial infections (Oechslin et al., 2016). There is some evidence that there is a trade-off between phage resistance and antibiotics resistance (Mangalea et al.,2020).
Although phage therapy faces many challenges ahead, the potential it has warrants further studies and research. If we manage to harness the therapeutic ability of phages, we can confidently face the threat of AMR head on. With this powerful weapon at our disposal, phage therapy will truly be able to usher in a new era of medicine. Bacterial infections may then finally become a thing of the past.
References:
Landecker, H. (2016). Antibiotic Resistance and the Biology of History. Body & Society, 22(4), 19–52. https://doi.org/10.1177/1357034X14561341
Mangalea, M. R., & Duerkop, B. A. (2020). Fitness Trade-Offs Resulting from Bacteriophage Resistance Potentiate Synergistic Antibacterial Strategies. Infection and immunity, 88(7), e00926-19. https://doi.org/10.1128/IAI.00926-19
Oechslin, F., Piccardi, P., Mancini, S., Gabard, J., Moreillon, P., Entenza, J. M., . . . Que, Y.-A. (2016). Synergistic Interaction Between Phage Therapy and Antibiotics Clears Pseudomonas Aeruginosa Infection in Endocarditis and Reduces Virulence. The Journal of Infectious Diseases, 215(5), 703-712. doi:10.1093/infdis/jiw632