Under Pressure: Hyperbaric Oxygen for Treating Carbon Monoxide Poisoning
Fields covered: Biomedical Science, Pressure
Related Articles:
§ ![]() Therapeutic effects of hyperbaric oxygen: integrated review §
Therapeutic effects of hyperbaric oxygen: integrated review § ![]() Hyperbaric Treatment Of Carbon Monoxide Toxicity
Hyperbaric Treatment Of Carbon Monoxide Toxicity
Carbon Monoxide (CO) poisoning occurs when someone is exposed to high amounts of CO in the environment. This can either be unintentional or intentional, since some people work near sites with high CO concentration or have chosen suicide by carbon monoxide poisoning.
The mechanism of CO poisoning in short works like this. CO is inhaled and dissolved into the bloodstream and it directly competes with existing dissolved Oxygen molecules (O2) to bind to haemoglobin (Hb). A refresher, haemoglobin is the molecule that exist in the red blood cells to transport O2 around the body. Since CO has about 250 times higher affinity to Hb as compared to O2, long exposure to CO would eventually lead to hypoxia (insufficiency of oxygen to cells and tissues) and then death.

The main way to treat CO poisoning is by administering 100% oxygen for long periods of time since oxygenation is the main way in which to eliminate CO from the body. However, doing this treatment at 1 atm (surface atmospheric pressure) is slow, hence people opt for Hyperbaric Oxygen Therapy.
Firstly, to the uninitiated, hyperbaric means high pressure environment and Hyperbaric Oxygen Therapy means being administered 100% Oxygen in a high-pressure environment.
Hyperbaric Oxygen Therapy works based on basic gas laws, specifically Henry’s law. It states that the amount of a given gas dissolved in a liquid is directly proportional to the partial pressure of the gas. In order to visualise this, imagine a sealed can of Coca-Cola. When sealed, there is high pressure in the can and most of the gases are dissolved in the drink. After opening, the pressure in the can reduces as gasses escapes, and air bubbles can be seen in the liquid escaping.
So, at high pressures, more O2 is dissolved in the bloodstream, effectively getting rid of more CO in the bloodstream.
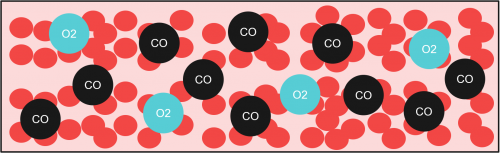
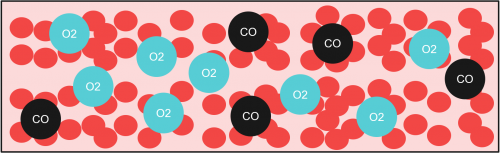
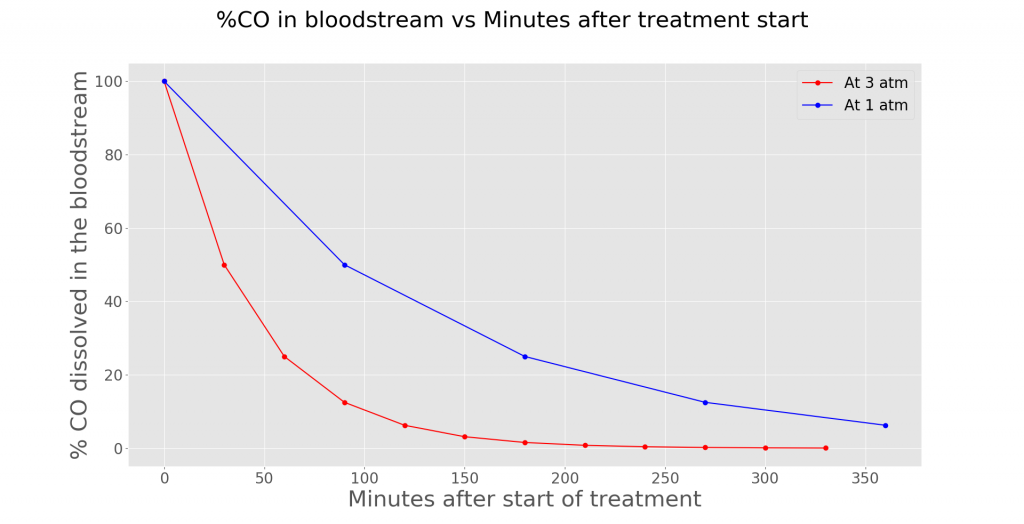
The immediate effects of this treatment include relieving hypoxia symptoms as more O2 is dissolved in the plasma (another portion of your blood). Moreover, CO poisoning in a hyperbaric environment is faster as compared to a non-hyperbaric environment (3 atm vs 1 atm). By investigating the half-life of CO at 1 atm and 3 atm in the bloodstream, a graph can be plotted.
Assuming that the patient is breathing 100% O2, the %CO dissolved in the bloodstream at 1 atm decreases slower than the %CO dissolved at 3 atm. Hence, this shows the positive effects of HBOT in treating CO.